Laurie Goodrich is an orthopedic surgeon whose typical patient weighs between 900 and 1,200 pounds. Her OR contains a gantry crane that lifts the anesthetized patient from the hallway floor and swings them gently to rest on an operating table big enough to suit a conference room. Her patients are horses. I met Laurie many years ago at the conclusion of a “Cowboy Horse Trailer Wreck” debacle, that you can read here.
Today, Dr. Goodrich is a professor in the College of Veterinary Medicine and Biomedical Sciences and Director of the Orthopedic Research Center at CSU’s C. Wayne McIlwraith Translational Medicine Institute (TMI). If you stop by the TMI off Drake Road in Ft. Collins, she will show you one by one preserved leg, ankle, and toe bones from horse surgeries she’s performed throughout her career. Although the metal plates and screws on these ivory colored bones are snug and secure, the surgeries themselves ended in failure, as the owner of these bones is obviously dead.
The most common reason that Dr. Goodrich’s operations aren’t successful is not that the screws and plates failed – it’s that the horse gets severe laminitis in the supporting, non-surgical foot. Laminitis is characterized by swelling, inflammation, and loss of blood flow to the area where the hoof attaches to bone—the equivalent of our nail bed.

Even after a successful surgery to repair the fracture, if the limb is tender and sore, the horse won’t bear any weight on it. Instead, it shifts its weight onto the contralateral supporting limb. The horse never takes the weight off this good foot, cutting off the blood supply to that hoof. Without the oxygen and nutrients supplied by blood flow, the laminae, a Velcro-like support network that keeps the coffin bone suspended within the hoof capsule, become inflamed and dies. As laminitis progresses, the supportive network formed by the laminae collapses. The coffin bone separates completely from the hoof, and the weight of the horse forces the bone downward, sometimes clear through the sole of the hoof. It’s incredibly painful for the horse, and horses that develop laminitis are usually euthanized.
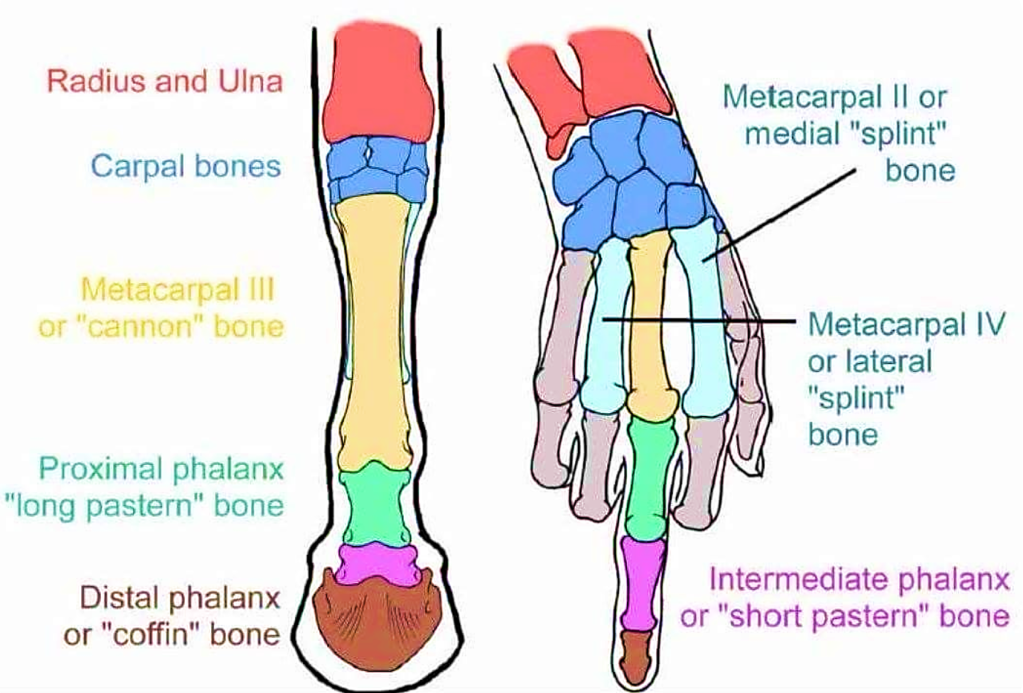
To appreciate what laminitis means to a horse, it helps to review the arrangement of the bones in a human hand and a horse’s leg. Horses are literally walking on the fingernail of their middle finger. The horse’s survival need to outrun predators drove the evolution of longer, lighter legs that could take longer strides with less energy. Horses on the grasslands didn’t need the side toes that kept their ancestors from rolling their legs during landing in the forest. The result was the evolution to a single long middle ‘toe’ that starts at the hock (or knee) with the third metatarsal, or cannon bone, and ends in the “nail” – the hoof. Over the last 20 million years, they have lost the other four digits from their hands and feet.
To get a personal experience with laminitis, just put your middle fingernail inside the lip of a drawer and then lean on it with several hundred pounds of pressure. Or pound the end of your finger into a wall with the same speed as you might throw a baseball. The biomechanical forces on the hoof of a horse are so extreme that it has evolved a unique laminar plexus of blood vessels where the hoof connects to the bone so that it doesn’t perpetually suffer the bruising and death of the hoof that humans call a black fingernail.

A schematic of the dynamics of equine hoof blood flow. The image on the right shows hoof blood flow when it is loaded with weight. Note that the blood does not perfuse the entire hoof. The image on the right shows hoof blood flow when the weight is unloaded. Note that the blood perfuses the entire hoof.
Source: https://www.theequinedocumentalist.com/post/supporting-limb-laminitis
This is the reason I have flown into Denver during a January blizzard and trekked up I-25 to visit Laurie in her office. For almost ten years, we have been dreaming of a medicine that would speed the regrowth of new bone after her surgeries, saving the significant number of horses that survive the operation but then die of support limb laminitis. Equine hoof blood flow is dependent on the ability to shift their body weight fully across all four limbs. Walking shifts the weight of the horse in a regular rhythm that maintains blood flow to the hoof, keeping it oxygenated, alive, and healthy. Each step cyclically loads and unloads weight on the hoof. When the horse steps down, the hoof is loaded with weight, and the increased pressure drives blood flow out of the hoof. When the horse lifts its foot, the weight is unloaded, the pressure is removed, and blood flow is restored to the hoof. When this rhythm ceases, so does the pumping action. The lack of pumping isn’t so bad for a short while. But bones take weeks to heal, and that’s where problems like laminitis occur.
Evolution is full of tradeoffs. A horse weighs a thousand pounds and carries all that weight on their middle toe. The pastern joint, which is analogous to a human’s ankle or wrist, is the natural weak spot in the horse, and arthritis in this joint is a common problem seen in working horses. Dr. Goodrich performs between 15 and 20 pastern joint fusions per year. These are mainly working pleasure horses that have developed lameness and arthritis in the articulation that is the second knuckle in your middle finger. She opens this joint, grinds out all the cartilage and debris and then fuses the two pastern bones together using steel plates and screws. If she can get new bone to grow across this space quickly enough, before laminitis attacks the opposite leg, the horse will enjoy another 7 to 10 years of comfortable riding instead of euthanasia. It all depends on the speed of new bone growth.
The sooner a horse can resume bearing weight on all four limbs, the better. Dr. Goodrich thinks just a 20 or 30% increase in the rate of mineralization of the repair will help improve the success rates of her operations. What equine surgeons like Dr. Goodrich need are therapies that promote bone regeneration to enhance healing. Metal plates and screws can be used to surgically fix fractures, fuse joints, and stabilize bones. But even metal hardware installed by the best surgeon will never support the weight of a horse if the bone does not fill in around it.
The answer to Laurie’s prayers is a selective activator of EP4, the receptor on bone growing osteoblast cells that instructs them to turn on and deposit new bone. It’s called KMN-159.

In the late 1970s in Kalamazoo, Michigan the Upjohn Company (bought by Pharmacia, then bought by Pfizer) was hoping to develop Prostaglandin E1 (PGE1) as a drug to treat hypertension. During animal studies, they performed weeks-long IV infusions of this substance into a group of beagles, continuously monitoring blood pressure. At the end of the study a full examination of all tissues revealed an unexpected doubling in the thickness of their leg bones. So, proof of concept for prostaglandins of the E-series to grow bone is almost 50 years old. It has also been shown more recently in humans, because although it failed as an anti-hypertensive, PGE1 is approved as a vasodilator in a rare neonatal population with severe cardiac defects. (It is also approved for direct injection into the penis to treat erectile dysfunction, but that kind of boner isn’t of interest in orthopedics.) The ductus arteriosus is a fetal blood shunt that must close during the transition to air breathing at birth. Failure of the vessel to close immediately isn’t that uncommon, but in those infants whose pulmonary artery and aorta are attached transposed to the wrong chambers of the heart, failure of ductus closure is the only thing keeping them alive. PGE1 (AlprostadilTM) is infused continuously via a cardiac catheter in these desperately ill babies in order for them to recover from birth and gain a few ounces of weight prior to the open cardiac surgery required to correct their vascular plumbing. And lo and behold — radiologists looking at chest films of these babies have often reported “unexpected increased radiodensity of the humerus bilaterally” or some such jargon meaning, “This kid is growing some Neanderthal style arm bones.”
It’s unusual that a potential new therapy this well documented sits undeveloped for 50 years. But Cayman Chemical, Maxey Appys and a new spin-out company Good Bone Newco* plan to change that. KMN-159 is a potent, stable, selective agonist of the EP4 receptor that solves the problems inherent in PGE1, those being chemical instability and non-selectivity. It also severely disrupts the only other agent on the market for growing bone. That agent is a biologic called Bone Morphogenic Protein (BMP, trade name InfuseTM) sold by Medtronic. Google Medtronic Lawsuit to find ample documentation of some shortfalls of InfuseTM. But suffice it to say, it is expensive to make, unstable and difficult to store, difficult to place surgically and, worst of all, grows ectopic bone: Bone where bone isn’t supposed to be. By contrast, KMN-159 is cheap to make, stable as a rock and can sit for years at room temperature, easily placed as formulated in a putty of collagen fibers, and it does not grow ectopic bone.
Dr. Goodrich is now joining forces with the team that will drive KMN-159 to completion as both a veterinary and human therapeutic.
Three former Pfizer chemists and biologists Jim O’Malley, Tom Owen, and Steve Barrett are the legacy inventors of this idea. Andrei Kornilov is the talented synthetic chemist who introduced the 2 fluorine atoms, twisting the lactam ring ever so slightly. Ines Moreno has supported the program and our academic collaborators with non-dilutive SBIR funding.
A CEO who can direct our project through clinical trials and raise the necessary funding is currently being recruited.
Clearly, horse pasterns are only a first step for Good Bone Newco*.There are also unmet needs in the repair of complex fractures in companion animals like dogs and cats. Moving on to humans, there are a variety of demands for newer and better bone growing therapeutics. We are working with an orthopedic surgeon in Europe to explore KMN-159 in human lumbar spinal fusion surgery. We have a collaboration with the University of Michigan school of Dentistry to explore the promotion of alveolar bone growth so that people needing a dental implant can grow enough new jaw bone to support it. Open heart surgery patients find that one of the most painful aspects of their recovery is the grinding of the cut edges where their sternum was sawed in half, and KMN-159 could speed the knitting of that bone to hasten return to normal life. There is hip replacement. Knee replacement. Osteoporosis. It’s hard to bring up our new bone growing product in front of an orthopedic surgeon without sparking a lot of enthusiasm and more new surgical applications. To be honest, horse pasterns weren’t the first thing we had on our minds when we invented KMN-159, but it seems to be the way this product will leap onto the orthopedic drug discovery stage.
Thanks to Karla Yurgaites for help with the editing and illustrations, and to Melissa Parsey for a substantial block of first-draft composition and research into equine evolution.
*Good Bone Newco is a terrible name. Leave your best suggestion for a name for this company in the Comments Section. If you would like to support this life saving improvement in the health care of both horses and humans, consider donating to C.A. Maxey Appaloosa Heritage Foundation. You may also communicate your other ideas for support to the lead author @KirkMMaxey in a Twitter DM.




What a great article about the forefront of medicine and the translational nature between horses and other species!
Thanks Meaghan! Somehow I didn’t find a place in the article to describe Dr. Goodrich’s splint bone model of non-junction long bone fracture. The fact that these bones bear no weight means that you can just saw a little gap in one of them and watch to see if it grows back, while the horse bears weight normally. We hope to include this study in a new grant by the end of the year.